Background: Thalassemias are a group of inherited blood disorders caused by impaired production of the globin chains of hemoglobin (Hb), resulting in anemia. HbE/β-thalassemia results from the co-inheritance of a specific point mutation in the β-globin gene with a second β-thalassemia mutation. The combination of mutations influences disease severity, ranging from mild to severe, with HbE/β-thalassemia accounting for about half of all cases of severe thalassemia. Patients (pts) with severe disease have higher red blood cell (RBC) transfusion requirements, which can be associated with increased risk of related morbidities. Treatments that can reduce dependence on RBC transfusions are needed to improve pt outcomes.
Primary results from the BELIEVE study (NCT02604433) demonstrated the efficacy and safety of luspatercept to treat anemia in transfusion-dependent pts with β-thalassemia across genotypes including HbE (Cappellini MD, et al. HemaSphere 2020;4[S1]:108-109). A subsequent analysis of the final BELIEVE data for pts with β 0/β 0 mutations, a form of severe β-thalassemia, demonstrated similar results with longer treatment in this subgroup (Sheth S, et al. Blood 2022;140 [S1]:1946-1948). The aim of this post hoc sub-analysis was to investigate the efficacy and safety of longer-term luspatercept treatment in pts in the BELIEVE trial with HbE/β-thalassemia.
Methods: The BELIEVE trial included adult pts with β-thalassemia or HbE/β-thalassemia (compound heterozygous β-thalassemia with mutation and/or multiplication of α-globin genes allowed) who required regular RBC transfusions (6-20 RBC units in the 24 wk before randomization with no transfusion-free period > 35 days). Pts were randomized 2:1 to receive luspatercept (1.0-1.25 mg/kg) or placebo subcutaneously every 3 wk and evaluated for response (defined as reduction in RBC transfusion burden ≥ 33% or ≥ 50% from baseline during any 12-wk interval over the entire study) and safety. Data were evaluated up to the final pt visit (Jan 5, 2021).
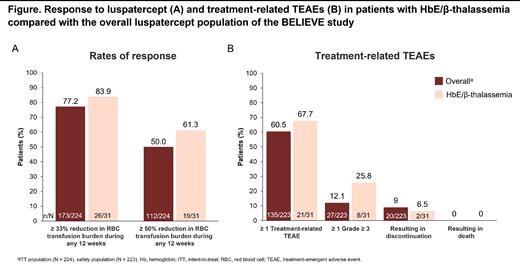
Results: The BELIEVE trial enrolled 336 pts, 52 (15.5%) of whom had HbE/β-thalassemia; 31 were randomized to the luspatercept group of the overall intent-to-treat (ITT) population (13.8%; N = 224). At baseline, pts receiving luspatercept with HbE/β-thalassemia were similar in age compared with the overall ITT population (median 32 y vs 30 y, respectively) and had similar transfusion burden (median 13.0 units vs 14.0 units over 24 wk), but had higher median fetal Hb (6.1% vs 5.1%), higher median serum ferritin (2087.0 vs 1441.3 μg/L), and higher median liver iron content (10.8 vs 6.1 mg/g dry weight). The median (range) duration of luspatercept treatment for pts with HbE/β-thalassemia was similar to the overall ITT population (157.7 [51.0-207.1] wk vs 153.6 [1.7-215.0] wk). A similar proportion of pts with HbE/β-thalassemia as in the overall ITT population achieved ≥ 33% reduction in transfusion burden (83.9% vs 77.2%) and ≥ 50% reduction in transfusion burden (61.3% vs 50.0%) over any 12-wk interval with luspatercept treatment (Figure A). Median total duration of ≥ 33% reduction in transfusion burden response was longer for HbE/β-thalassemia pts versus the overall ITT population (722.0 days vs 586.0 days).
In the HbE/β-thalassemia subgroup, 67.6% of pts experienced ≥ 1 treatment-emergent adverse event (TEAE) related to treatment versus 60.5% of pts in the overall luspatercept safety population. The proportion of pts experiencing grade ≥ 3 treatment-related TEAEs was 25.8% in the HbE/β-thalassemia subgroup and 12.1% in the overall safety population. Treatment-related TEAEs resulted in discontinuation of luspatercept in 6.5% and 9.0% of the HbE/β-thalassemia and overall safety populations, respectively. No treatment-related TEAEs resulted in death in either group (Figure B).
Summary: The HbE/β-thalassemia sub-group of the BELIEVE study had rates of response that were consistent with the larger ITT population. Furthermore, pts with HbE/β-thalassemia experienced a longer median total duration of response compared with the overall ITT population with a similar safety profile. Additional evaluation of the efficacy and safety of luspatercept in a larger population of HbE/β-thalassemia pts would be valuable as the number of pts in this sub-analysis was small, yet these results suggest that luspatercept is as safe and effective for this subgroup of pts as for the wider β-thalassemia population.
Disclosures
Kuo:Agios Pharmaceuticals: Consultancy, Research Funding; Forma Therapeutics: Consultancy; Pfizer: Consultancy; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Sangamo: Membership on an entity's Board of Directors or advisory committees; Novo/Nordisk: Consultancy, Honoraria; Vertex Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy. Sutcharitchan:Faculty of Medicine Chulalongkorn University: Current Employment. Chew:Protagonist Therapeutics, Inc.: Research Funding; Bristol Myers Squibb: Research Funding. Goh:Penang Hospital: Current Employment; Bristol Myers Squibb: Research Funding. Vodala:Mabgenex: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Current Employment. Kuo:Bristol Myers Squibb: Current Employment. Lai:Bristol Myers Squibb: Current Employment. Felber Medlin:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Perin:Celgene, a Bristol Myers Squibb company: Current Employment, Current equity holder in publicly-traded company. Moro Bueno:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Coates:Chiesi: Consultancy; Bristol Myers Squibb: Consultancy; Agios Pharmaceuticals: Consultancy. Viprakasit:Bristol Myers Squibb: Research Funding; Siriraj Hospital: Current Employment; Novartis: Research Funding; Silence Co. Ltd.: Research Funding; GPO, Thailand: Research Funding.